Case of the month: February 2020 - losing the pot
About this case study
Each month JAG publishes a case of the month highlighting a real life clinical scenario which has impacted patient safety. Case studies are contributed by JAG leads and endoscopy services across the UK and are designed to provide an opportunity for discussion and to share learning.
Download the PDF version hereand share this for discussion with your team.
Case synopsis
A 56-year-old woman with a history suspicious for colorectal cancer had a failed colonoscopy. She underwent CT colonography which raised the possibility of an early cancer or suspicious polyp in the ascending colon. She was referred to a specialist centre for a repeat attempt at colonoscopy under general anaesthetic. The intubation was challenging due to sigmoid fixation.
The endoscope was eventually passed to the ascending colon and a lesion identified. In total, eight biopsies were taken. The patient recovered uneventfully and was discharged. The pathology samples were sent to the laboratory urgently and the endoscopy checklist was completed.
The patient returned to clinic 1 week later. The endoscopist was concerned that no pathology result was available on the pathology system. When the laboratory was called, the endoscopist was informed that no specimen was received. A labelled sample pot had been submitted to the laboratory but there was no tissue sample in the pot.
The endoscopist was told that the laboratory had contacted the endoscopy unit late in the day of the procedure to inform them. This message had not been relayed to the endoscopist.
The patient was informed of events (considering duty of candour) and had to be recalled for a further colonoscopy under general anaesthetic to obtain confirmatory biopsies. Further biopsies confirmed adenocarcinoma. An apology was given to the patient who had by now had four bowel preparations and four procedures to diagnose her bowel cancer.
JAG analysis
What are the learning points you took away from this case?
How could things have been improved?
The following patient safety incidents were identified by JAG. They have been categorised for severity (mild, moderate and severe) based on the actual or potential impact to the patient and adherence to clinical guidance, as well as by theme [2]:
| 1 | Oxygen monitoring |
| 2 | Distractors and time management |
| 3 | Non-technical skills and training |
| 4 | Documentation and reporting errors |
| 5 | Technical skills and equipment |
| 6 | Sedation intravenous access and monitoring |
| 7 | Drug errors |
| 8 | Consent |
| 9 | Histology and sampling errors |
| 10 | Other |
Patient safety incident | Categorical theme | Severity |
A sample wasn't received by laboratory | 9 | Severe |
| The message from pathology was not escalated to relevant staff | 3 | Mild |
Exposure to a repeat procedure including bowel preparation and general anaesthetic | 10 | Moderate |
| Ineffective use of the endoscopy safety checklist (sign out) | 3 | Moderate |
Did you identify any other patient safety incidents?
Learning
Histology pots should be double-checked by two members of staff, including the endoscopist.
All histology pots should be checked for:
1. Patient identification — using a three-point identification check (ie patient name, date of birth and hospital number)
2. Sample type clearly and legibly written (eg colonic lesion biopsies)
3. Date of specimen clearly and legibly written
4. Visual presence of sample in pot which represents that expected (eg eight samples on a strip)
5. Endoscopist engagement in ‘sign out’ section of checklist
During the procedure, closed loop communication should be used to confirm everyone is aware that samples have been taken. For example, the endoscopist states that eight colonic lesion samples have been taken and the nursing team reports back to endoscopist “eight samples taken."
Samples pots should be checked before the patient leaves the endoscopy room, as part of the ‘sign out’ Checklist procedure, where any problems can be immediately addressed [1].
Problems identified with pathology samples should be reported to the endoscopist at the earliest possible opportunity. The laboratory could have contacted the endoscopist directly or staff receiving the phone call from pathology could have escalated the incident to the unit manager and endoscopist. Recording this on an incident reporting system would facilitate inter-professional communication.
Other identified issues can occur with pathology samples in endoscopy. Here are some solutions to possible pathology errors in endoscopy:
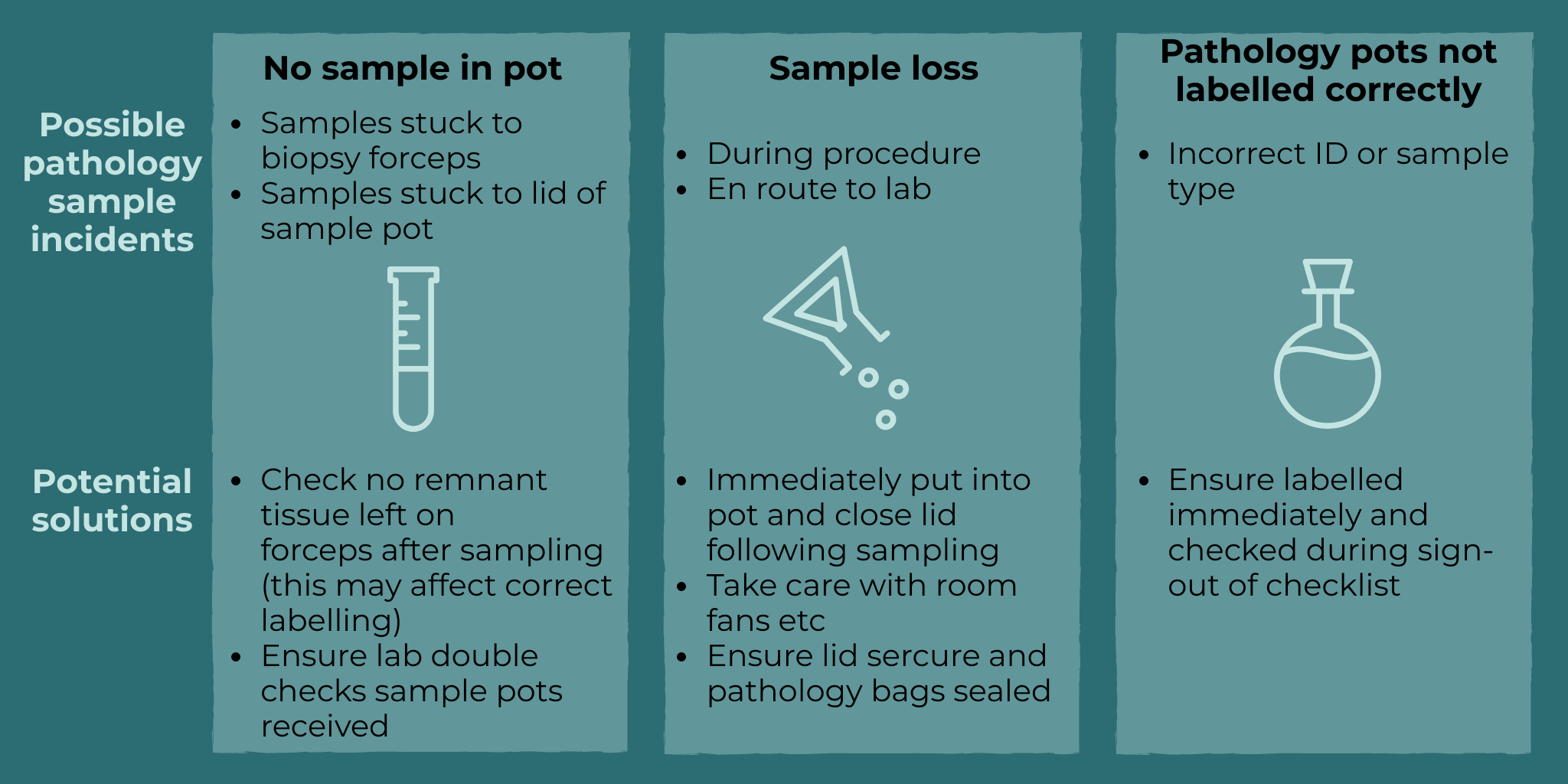
Services should have a standard operating procedure (SOP) regarding pathology acquisition. JAG recommends services review their SOPs in light of this case.
Do you have a case study from your service which you would like to share in the next ISREE case study of the month?
Please contact askjag@rcplondon.ac.uk for more information
This case is informed by real-life events and the narrative has been adapted to enhance educational benefit. No identifiable information has been provided.
References
1. Matharoo, M., et al., Implementation of an endoscopy safety checklist. Frontline Gastroenterology, 2014. 5(4): p. 260-265.