Case of the month: February 2021 - How clean is clean?
About this case study
Download the PDF version and share this for discussion with your team!
Case synopsis
A general medical inpatient developed a multidrug resistant Carbapenemase-resistant Enterobacteriaceae (CRE) Klebsiella pneumoniae, identified in sputum and blood culture. Sources of the infection were investigated. There was no other patient on the ward with this organism at the time. It was noted that this patient underwent an OGD 5 days previously. On further review, this procedure was undertaken with the same endoscope used on a patient previously diagnosed with Klebsiella p. The endoscope was removed from circulation and tested. Preliminary results show the same multidrug resistant pattern as the organism causing bacteraemia.
Learning
Carbapenemase-resistant Enterobacteriaceae (CRE)
- CRE are bacteria that can cause significant morbidity and mortality.
- Treatment options tend to be limited due to antibiotic resistance.
- Transmission of CRE has been linked to endoscope contamination and is rare. There is no evidence to suggest that this is due to resistance to commonly used disinfectants. No extra decontamination steps are required for patients colonised or infected with CRE. Transmission can occur due to the reasons outlined below.
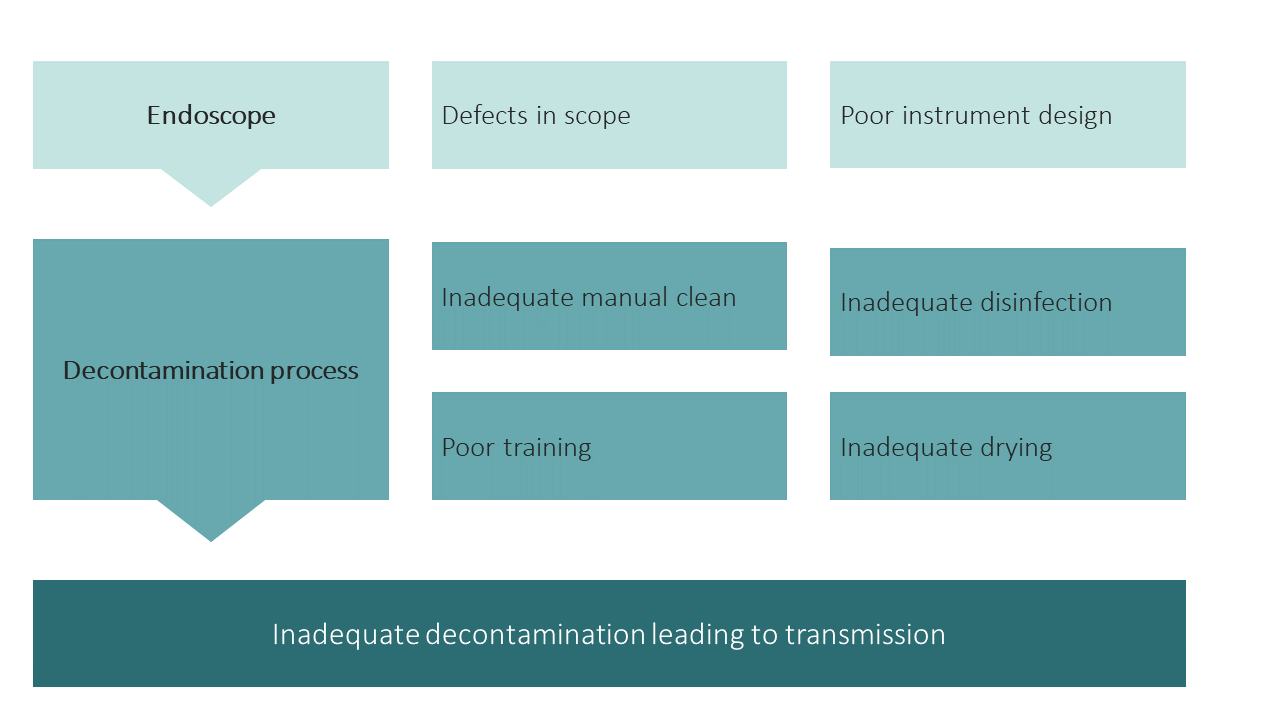
- Decontamination should be a whole team responsibility, ranging from all individuals who use or handle the scope to those that maintain and repair equipment. Any error along this pathway could contribute to inadequate decontamination and subsequent patient safety issues.
Tracking and traceability
- The risk of transmitting infection by endoscopy is very low, however all units should have a process for tracking equipment used during each procedure if an infection is found.
- The 2020 BSG Decontamination guidance provides an overview of this process:
- Serial numbers of all endoscopes and accessories must be recorded for each patient examined and tracked throughout the decontamination processes.
- Tracking of the personnel undertaking each step of the decontamination process and the patient associated with each endoscope and all its accessories should be undertaken using an electronic method.
- The detachable components should be kept with their corresponding endoscope, forming a unique set.
- There must also be a means of tracking for each patient use of any reusable endoscopy accessories.
- A record of the decontamination process should be retained.
- It should be possible to demonstrate that an endoscope has been through a full reprocessing cycle prior to use in any new patient. This entire process should be subject to regular audit.
- Endoscopes should be regularly maintained and serviced and replaced once obsolete. There should be a process to monitor the maintenance, servicing, and replacement of all scopes.
- Endoscopy performed outside normal working hours/ off unit need to follow the same processes.
Training
- JAG recommends the use of JETS Workforce to support competency development and training.
- All staff should have a DOPS training and assessment tool for manual cleaning processes in endoscopic decontamination. This should then form part of annual appraisal and revalidation of practice.
The risks and benefits of endoscopy form an important part of the pre-assessment process. This is even more carefully scrutinised as a result of COVID-19 to ensure patients who need endoscopy have it safely and alternative investigations are identified for other patients.
What are your views on this case? Continue the discussion online @JAG_Endoscopy #COTM Have you had any learning points with similar experiences that you wish to share with endoscopy community? Contact askjag@rcplondon.ac.ukfor more information. |